CHM 1020 Chapter Nine Study Packet - Part VI
|
Chapter Nine:
Chemical Kinetics - Chapter 9 |
|
|
|
|
|
G8 Factors forming the
rate Constant- Answer |
|
|
G9: Free Radical Mechanism of Halogenation of Alkane
(lecture) Answer |
|
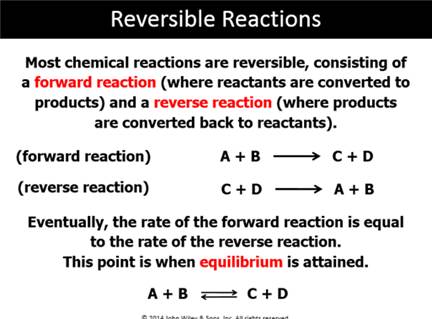
Part G7: Law of Mass Action
In the reaction: A + B ------> C + D ; assuming the reaction is first order with respect to both the reactants A and B;
the rate expression is: Rate = k’[A][B]. The reverse reaction is not a factor in the initial concentration changes.
Demonstrate the Law of Mass Action in explaining why you
multiple the concentrations A times the
concentration of B in the rate expression
( [A][B] ) .
What does this
product represent?
Is this product a
number greater or less than one?
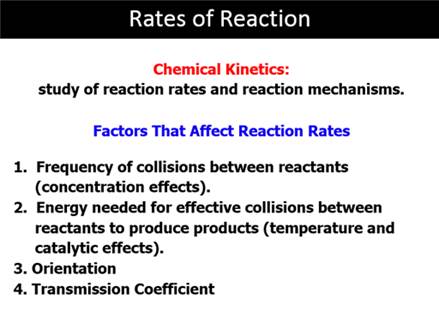
Part G8: Factors Forming Rate Constant: k’
In the Rate expression in Part A: RateINT = dP/dT = k’[A][B];
Is the rate constant
k’ a number greater than one or less than one, why?
What does this number represent?
The rate constant k’ can be broken down into the product of four factors. What are the four factors and what does each factor represent? Are each of these greater than one of less than one?
1)
2)
3)
4)
Part G9: Free Radical Mechanism Example
The free radical mechanism for the halogenation of alkane has been established as a chain reaction. Demonstrate this mechanism by showing all four steps in this mechanism using Methane and Bromine in Ultraviolet Light
CH4 + Br2 + uv light à CH3Br + HBr
a.
Chain initiation step:
b.
Chain Propagation Steps
c.
Chain Termination
Steps:
d.
What evidences did the
chemist record which lead to this suggested mechanism?